 )
) )
)Hover over a description to show an image; click on the blue links to view the R code.
Links to literature references show sources of data, equations, or original diagrams.
Can't find the demo you're after? There are more demos .
.
 (Wagner and Pruss, 2002)
(Wagner and Pruss, 2002)
 (Shock et al., 1992)
(Shock et al., 1992)
 , copper
, copper , and manganese
, and manganese (Pourbaix, 1974)
(Pourbaix, 1974)
 (Garrels and Christ, 1965)
(Garrels and Christ, 1965)
 and gold
and gold (cf. Skirrow and Walshe, 2002)
(cf. Skirrow and Walshe, 2002)

 and sulfate
and sulfate (Migdisov et al., 2024)
(Migdisov et al., 2024)

 (Lu and Zhu, 2011)
(Lu and Zhu, 2011)
 (Stumm and Morgan, 1996)
(Stumm and Morgan, 1996) and calcite
and calcite (Manning et al., 2013)
(Manning et al., 2013)
 (Akinfiev and Zotov, 2001
(Akinfiev and Zotov, 2001 ; Stefánsson and Seward, 2004
; Stefánsson and Seward, 2004 ; Williams-Jones et al., 2009
; Williams-Jones et al., 2009 )
)  and optional data
and optional data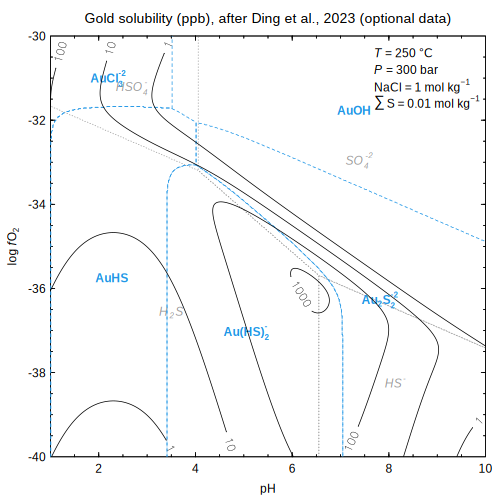 (Ding et al., 2023
(Ding et al., 2023 ; Tagirov et al., 2024
; Tagirov et al., 2024 )
) (Akinfiev and Tagirov, 2014
(Akinfiev and Tagirov, 2014 )
) (cf. Spinks et al., 2021)
(cf. Spinks et al., 2021)
 (Bowers et al., 1984)
(Bowers et al., 1984)
 (Sverjensky et al., 2014a
(Sverjensky et al., 2014a , 2014b)
, 2014b)
 (Sverjensky et al., 1991)
(Sverjensky et al., 1991)
 (Tutolo et al., 2014
(Tutolo et al., 2014 ; Zimmer et al., 2016
; Zimmer et al., 2016 )
) , volume
, volume , and heat capacity
, and heat capacity (Akinfiev and Diamond, 2003)
(Akinfiev and Diamond, 2003)
 —
— logB.to.OBIGT() fits at 800 and 1000
and 1000 bar and Y-Cl speciation
bar and Y-Cl speciation in
in NaCl() solution (Guan et al., 2020)